Pushing Electrons Daniel P Weeks Pdf Editor
Pushing Electrons 4th Edition by Weeks, Daniel P. Textbook PDF Download Data bank archived file. Download link: File name: Pushing.Electrons.4th.Edition.by.Weeks.Daniel.P.Textbook.PDF.Download.Data.bank.PDF Read online Pushing Electrons 4th Edition by Weeks, Daniel P. Textbook PDF Download Data bank Buy Pushing Electrons 4th Edition by Weeks, Daniel P. Textbook PDF Download Data bank Download and read Pushing Electrons 4th Edition by Weeks, Daniel P. Textbook PDF Download Data bank ebook, pdf, djvu, epub, mobi, fb2, zip, rar, torrent Download to iPad/iPhone/iOS, B&N nook Pushing Electrons 4th Edition by Weeks, Daniel P.
Textbook PDF Download Data bank ebook, pdf, djvu, epub, mobi, fb2, zip, rar, torrent Pushing Electrons 4th Edition by Weeks, Daniel P. Textbook PDF Download Data bank Solution Manual / BrainDump / Testbank Report South Korea May Ask US to Re-Deploy Its Nuclear US and South Korean forces are definitely technically capable of decimating the North Korean military with conventional methods alone, though not without weeks or Congressional Hearing About Net Neutrality Postponed The Federal Communications Commission has been pushing for a rollback of Obama-era net neutrality protections that prevent internet service providers from selectively Google Even more Account Options. Sign in Search settings Google Search the worlds information, including webpages, images, videos and more. Google has many special features to help you find exactly what youre looking for. Statistical Techniques Statistical Mechanics Statistical Techniques Statistical Mechanics httpdilefd.ruam.html efadqwe32e.
This article needs additional citations for. Unsourced material may be challenged and removed. (April 2009) () Arrow pushing or electron pushing is a technique used to describe the progression of mechanisms. It was first developed. In using arrow pushing, 'curved arrows' or 'curly arrows' are superimposed over the of reactants in a to show the. The arrows illustrate the movement of as between are broken and formed.
Arrow pushing is also used to describe how positive and negative are distributed around through. It is important to always remember, however, that arrow pushing is a formalism and electrons (or rather, electron density) do not move around so neatly and discretely in reality.
Recently, arrow pushing has been extended to, especially to the chemistry of s- and p- elements. It has been shown to work well for compounds. Trajectory of single electron When a bond is broken, electrons leave where the bond was and this is represented by a curved arrow pointing away from the bond and ending the arrow pointing towards the next unoccupied molecular orbital. Similarly, organic chemists represent the formation of a bond by a curved arrow pointing between two species. For clarity, when pushing arrows, it is best to draw the arrows starting from a lone pair of electrons or filled bonds (sigma, pi) and ending in an unfilled molecular orbital, allowing the reader to know exactly which electrons are moving and where they are ending. Breaking of bonds [ ] A joining atoms in an organic molecule consists of a group of two electrons.
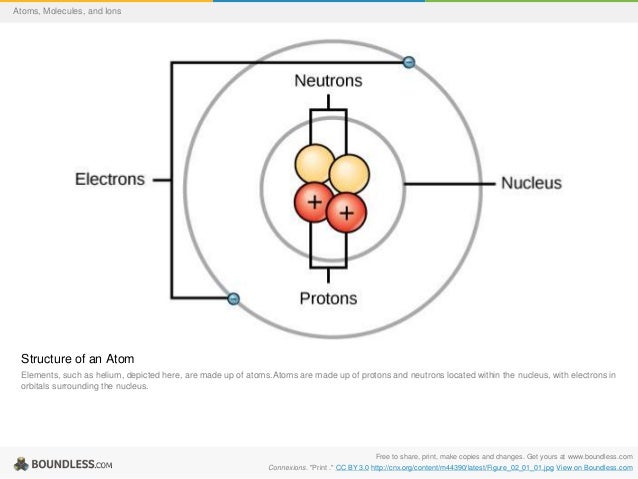
Such a group is referred to as an electron pair. Reactions in organic chemistry proceed through the sequential breaking and formation of such bonds. Organic chemists recognize two processes for the breaking of a chemical bond. These processes are known as homolytic cleavage and heterolytic cleavage. Homolytic bond cleavage [ ] Homolytic is a process where the electron pair comprising a bond is split, causing the bond to break. This is denoted by two single barbed curved arrows pointing away from the bond. The consequence of this process is the retention of a single unpaired electron on each of the atoms that were formerly joined by a bond.
Pushing Electrons (889): Daniel P. To teach you to properly push electrons to generate resonance. Pushing Electrons by Daniel P.
These single electron species are known as. For example, light causes the -chlorine bond to break homolytically. This is the initiation stage of. Heterolytic bond cleavage [ ] Heterolytic bond cleavage is a process where the electron pair that comprised a bond moves to one of the atoms that was formerly joined by a bond. The bond breaks, forming a negatively charged (an ) and a positively charged species (a ). The anion is the species that retains the electrons from the bond while the cation is stripped of the electrons from the bond. The anion usually forms on the most atom, in this example atom A.
Heterolytic reaction mechanisms [ ] All heterolytic organic chemistry reactions can be described by a sequence of fundamental mechanistic subtypes. The elementary mechanistic subtypes taught in introductory organic chemistry are S N1, S N2, E1, E2, addition and addition-elimination. Using arrow pushing, each of these mechanistic subtypes can be described. S N1 reactions [ ] An occurs when a molecule separates into a positively charged component and a negatively charged component.
This generally occurs in highly polar through a process called. The positively charged component then reacts with a forming a new compound.
Step 2, substitution. Drum Kits Wav Download Free more. In the first stage of this reaction (solvolysis), the C-L bond breaks and both electrons from that bond join L (the ) to form L − and R 3C + ions. This is represented by the curved arrow pointing away from the C-L bond and towards L. The nucleophile Nu −, being attracted to the R 3C +, then donates a pair of electrons forming a new C-Nu bond. Because an S N1 reaction proceeds with the Substitution of a leaving group with a Nucleophile, the S N designation is used.
Because the initial solvolysis step in this reaction involves a single molecule dissociating from its leaving group, the initial stage of this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to S N1.
S N2 reactions [ ] An occurs when a nucleophile displaces a leaving group residing on a molecule. This displacement or substitution results in the formation of a new compound. E1 eliminations proceed with the Elimination of a leaving group leading to the E designation.
Customer Programming Software Cps Download For Mac more. Because this mechanism proceeds with the initial dissociation of a single starting material forming a carbocation, this process is considered a uni-molecular reaction. The involvement of only 1 species in the initial phase of the reaction enhances the mechanistic designation to E1. E2 eliminations [ ] An E2 elimination occurs when a proton adjacent to a leaving group is extracted by a with simultaneous elimination of a leaving group and generation of a double bond. E2 eliminations proceed through initial extraction of a proton by a base or nucleophile leading to Elimination of a leaving group justifying the E designation. Because this mechanism proceeds through the interaction of two species (substrate and base/nucleophile), E2 reactions are recognized as bi-molecular.
Thus, the involvement of 2 species in the initial phase of the reaction enhances the mechanistic designation to E2. Addition reactions [ ] occur when nucleophiles react with. When a nucleophile adds to a simple or, the result is a 1,2-addition. When a nucleophile adds to a conjugated carbonyl system, the result is a 1,4-addition. The designations 1,2 and 1,4 are derived from numbering the atoms of the starting compound where the oxygen is labeled “1” and each atom adjacent to the oxygen are sequentially numbered out to the site of nucleophilic addition. A 1,2-addition occurs with nucleophilic addition to position 2 while a 1,4-addition occurs with nucleophilic addition to position 4.